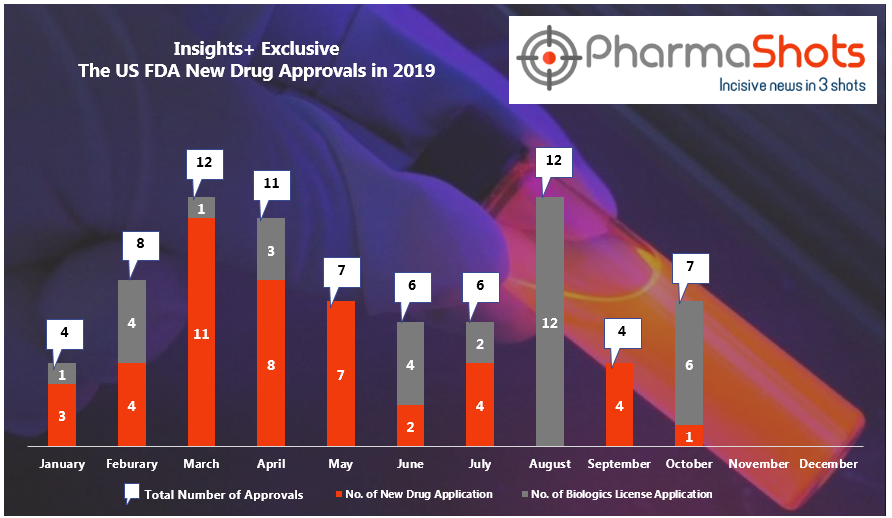
Ardelyx Reports the NMPA Acceptance of NDA for Tenapanor to Treat Hyperphosphatemia in China
Shots:
- The NMPA has accepted the NDA for tenapanor to control serum phosphorus in adult patients with CKD on hemodialysis
- Under the terms of the license agreement b/w Ardelyx & its cooperation partner Fosun Pharma, this acceptance resulted in ~$2M milestones to Ardelyx. By the end of 2024, it's anticipated that China will approve the NDA filing
- Ardelyx received $12M up front, ~$110M in developmental & commercialization milestones along with royalty while Fosun Pharma gets an exclusive right to market and sell tenapanor in China, Hong Kong & Macau. Ardelyx collaborated with Kyowa Kirin in Japan, Fosun Pharma in China & Knight Therapeutics in Canada for the development & commercialization of tenapanor in their respective territories
Ref: Ardelyx | Image: Ardelyx
Related News:- Ardelyx Amends 2017 Agreement with Kyowa Kirin for Tenapanor to Treat Hyperphosphatemia in Japan
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.